Here is an in-depth look at silver the element: Basic Information: Symbol: Ag Atomic number: 47 Group number: 11 Mass: 107.868 Density @ 293 K: 10.5 g/cm3 Atomic volume: 10.3 cm3/mol. Silver (Ag) is a silver metal that has the atomic number 47 in the periodic table. It is a Transition metal and located in Group 11 of the periodic table. It has the symbol Ag. Silver is a metal that has been known since prehistoric times and its discoverer are unknown.
We remember from our school chemistry course that every element has its own specific atomic number. It is the same as the number of protons that the atom of each element has, so sometimes atomic number is called proton number. It is always the whole number and it ranges from 1 to 118, according to the number of the element in the Periodic Table. This number can be really important and something essential to know, in relation to a certain chemical element which is the issue of our interest at the moment.
Why is this so? Why is the atomic number so important? First of all, it is the number that makes elements different from one another as it shows the number of protons in their nuclei. Also, knowing the atomic number of an element can give us an idea about the position of the element in the Periodic Table. Atomic number of an element never changes: for example, the atomic number of oxygen is always 8, and the atomic number of Chlorine is always 18. The atomic number is marked with the symbol Z, taken from a German word zahl (or atomzahl, which is 'atomic number' in German).
This website is created for those who need to know the atomic number of a central chemical element. By using our website, you can do it in just one click and receive short and correct information on this matter. There is also some extra summary on every each chemical element which can be found at our website, including the atomic weight of each element, as well as physical and chemical properties of every element and its importance. Use this website at any time when you need to get fast and precise information about atomic or proton number of chemical elements.
Atomic Number: 47: Atomic Radius: 172 pm (Van der Waals) Atomic Symbol: Ag: Melting Point: 961.78 °C: Atomic Weight: 107.9: Boiling Point: 2162 °C: Electron. Silver is a chemical element with the symbol Ag (from the Latin argentum, derived from the Proto-Indo-European h₂erǵ: 'shiny' or 'white') and atomic number 47. A soft, white, lustrous transition metal, it exhibits the highest electrical conductivity, thermal conductivity, and reflectivity of any metal. citation needed The metal is found in the Earth's crust in the pure, free elemental. Element is silver. Electronic configuration of the last shell is Kr 4d10 5s1. This means that the element is mono valent since the 5s1 electron is available for bonding rather than the paired 'd' electrons.
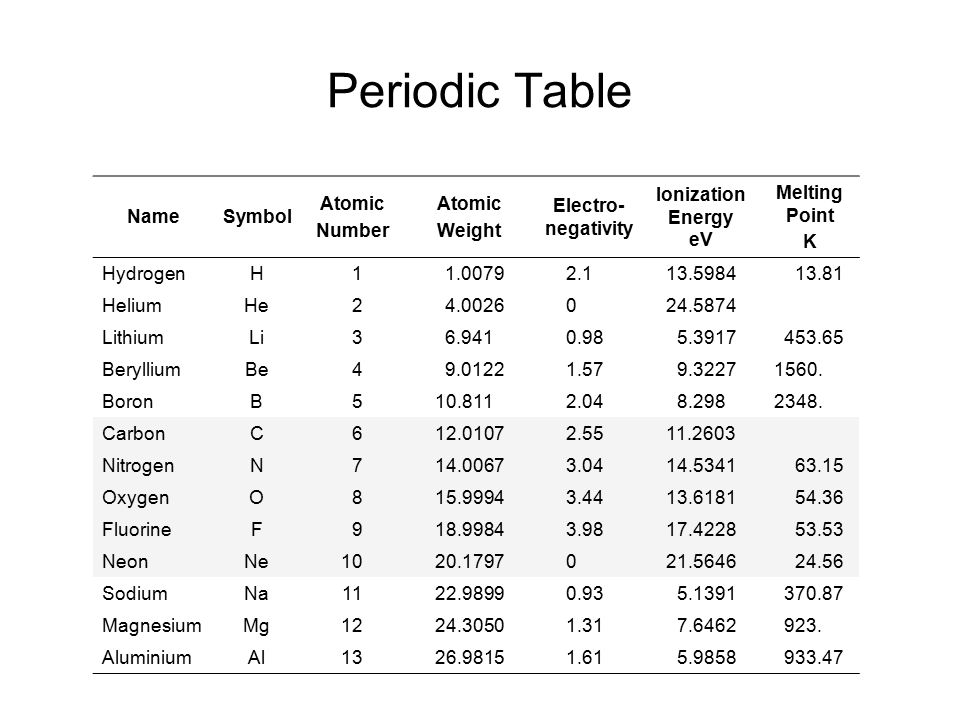
List of chemical elements in periodic table with atomic number, chemical symbol and atomic weight. You can sort the elements by clicking on the table headers. Please click on the element name for complete list of element properties.
| Atomic Number | Chemical Symbol | Element Name | Atomic Weight (u) |
|---|---|---|---|
| 1 | H | Hydrogen | 1.008 |
| 2 | He | Helium | 4.003 |
| 3 | Li | Lithium | 6.94 |
| 4 | Be | Beryllium | 9.012 |
| 5 | B | Boron | 10.81 |
| 6 | C | Carbon | 12.011 |
| 7 | N | Nitrogen | 14.007 |
| 8 | O | Oxygen | 15.999 |
| 9 | F | Fluorine | 18.998 |
| 10 | Ne | Neon | 20.18 |
| 11 | Na | Sodium | 22.99 |
| 12 | Mg | Magnesium | 24.305 |
| 13 | Al | Aluminium | 26.982 |
| 14 | Si | Silicon | 28.085 |
| 15 | P | Phosphorus | 30.974 |
| 16 | S | Sulfur | 32.06 |
| 17 | Cl | Chlorine | 35.45 |
| 18 | Ar | Argon | 39.948 |
| 19 | K | Potassium | 39.098 |
| 20 | Ca | Calcium | 40.078 |
| 21 | Sc | Scandium | 44.956 |
| 22 | Ti | Titanium | 47.867 |
| 23 | V | Vanadium | 50.942 |
| 24 | Cr | Chromium | 51.996 |
| 25 | Mn | Manganese | 54.938 |
| 26 | Fe | Iron | 55.845 |
| 27 | Co | Cobalt | 58.933 |
| 28 | Ni | Nickel | 58.693 |
| 29 | Cu | Copper | 63.546 |
| 30 | Zn | Zinc | 65.38 |
| 31 | Ga | Gallium | 69.723 |
| 32 | Ge | Germanium | 72.63 |
| 33 | As | Arsenic | 74.922 |
| 34 | Se | Selenium | 78.971 |
| 35 | Br | Bromine | 79.904 |
| 36 | Kr | Krypton | 83.798 |
| 37 | Rb | Rubidium | 85.468 |
| 38 | Sr | Strontium | 87.62 |
| 39 | Y | Yttrium | 88.906 |
| 40 | Zr | Zirconium | 91.224 |
| 41 | Nb | Niobium | 92.906 |
| 42 | Mo | Molybdenum | 95.95 |
| 43 | Tc | Technetium | 98 |
| 44 | Ru | Ruthenium | 101.07 |
| 45 | Rh | Rhodium | 102.906 |
| 46 | Pd | Palladium | 106.42 |
| 47 | Ag | Silver | 107.868 |
| 48 | Cd | Cadmium | 112.414 |
| 49 | In | Indium | 114.818 |
| 50 | Sn | Tin | 118.71 |
| 51 | Sb | Antimony | 121.76 |
| 52 | Te | Tellurium | 127.6 |
| 53 | I | Iodine | 126.904 |
| 54 | Xe | Xenon | 131.293 |
| 55 | Cs | Caesium | 132.905 |
| 56 | Ba | Barium | 137.327 |
| 57 | La | Lanthanum | 138.905 |
| 58 | Ce | Cerium | 140.116 |
| 59 | Pr | Praseodymium | 140.908 |
| 60 | Nd | Neodymium | 144.242 |
| 61 | Pm | Promethium | 145 |
| 62 | Sm | Samarium | 150.36 |
| 63 | Eu | Europium | 151.964 |
| 64 | Gd | Gadolinium | 157.25 |
| 65 | Tb | Terbium | 158.925 |
| 66 | Dy | Dysprosium | 162.5 |
| 67 | Ho | Holmium | 164.93 |
| 68 | Er | Erbium | 167.259 |
| 69 | Tm | Thulium | 168.934 |
| 70 | Yb | Ytterbium | 173.045 |
| 71 | Lu | Lutetium | 174.967 |
| 72 | Hf | Hafnium | 178.49 |
| 73 | Ta | Tantalum | 180.948 |
| 74 | W | Tungsten | 183.84 |
| 75 | Re | Rhenium | 186.207 |
| 76 | Os | Osmium | 190.23 |
| 77 | Ir | Iridium | 192.217 |
| 78 | Pt | Platinum | 195.084 |
| 79 | Au | Gold | 196.967 |
| 80 | Hg | Mercury | 200.592 |
| 81 | Tl | Thallium | 204.38 |
| 82 | Pb | Lead | 207.2 |
| 83 | Bi | Bismuth | 208.98 |
| 84 | Po | Polonium | 209 |
| 85 | At | Astatine | 210 |
| 86 | Rn | Radon | 222 |
| 87 | Fr | Francium | 223 |
| 88 | Ra | Radium | 226 |
| 89 | Ac | Actinium | 227 |
| 90 | Th | Thorium | 232.038 |
| 91 | Pa | Protactinium | 231.036 |
| 92 | U | Uranium | 238.029 |
| 93 | Np | Neptunium | 237 |
| 94 | Pu | Plutonium | 244 |
| 95 | Am | Americium | 243 |
| 96 | Cm | Curium | 247 |
| 97 | Bk | Berkelium | 247 |
| 98 | Cf | Californium | 251 |
| 99 | Es | Einsteinium | 252 |
| 100 | Fm | Fermium | 257 |
| 101 | Md | Mendelevium | 258 |
| 102 | No | Nobelium | 259 |
| 103 | Lr | Lawrencium | 266 |
| 104 | Rf | Rutherfordium | 267 |
| 105 | Db | Dubnium | 268 |
| 106 | Sg | Seaborgium | 269 |
| 107 | Bh | Bohrium | 270 |
| 108 | Hs | Hassium | 277 |
| 109 | Mt | Meitnerium | 278 |
| 110 | Ds | Darmstadtium | 281 |
| 111 | Rg | Roentgenium | 282 |
| 112 | Cn | Copernicium | 285 |
| 113 | Nh | Nihonium | 286 |
| 114 | Fl | Flerovium | 289 |
| 115 | Mc | Moscovium | 290 |
| 116 | Lv | Livermorium | 293 |
| 117 | Ts | Tennessine | 294 |
| 118 | Og | Oganesson | 294 |

Lists of Elements in Periodic Table
You can also list the elements in various ordered properties with printable tables below.
Lists of Elements by Group Number in Periodic Table
» Group 1» Group 2» Group 3» Group 4» Group 5» Group 6» Group 7» Group 8» Group 9» Group 10» Group 11» Group 12» Group 13» Group 14» Group 15» Group 16» Group 17» Group 18What is the electron configuration of Ag?
2 Answers
The electron configuration for silver (Ag) is based upon the place meant of silver in the fifth row of the periodic table in the 11th column of the periodic table or the 9th column of the transition metal or d block. Therefore th electron configuration for silver must end as
This notation can be written in core notation or noble gas notation by replacing the
For some of the transition metals they will actually transfer an s electron to complete the d orbital, making silver,
I hope this was helpful.
SMARTERTEACHER

Explanation:
Silver,
This tells you that a neutral silver atom will have a total of
Now, you have to be a little careful with silver because it is a transition metal, which implies that the occupied d-orbitals are actually lower in energy than the s-orbitals that belong to the highest energy level.
Atomic Number 47
So, here's how silver's electron configuration would look if it followed the Aufbau principle to the letter
Now, for the energy level
This means that you will have to switch the 3d orbitals on one hand, and the 4s and 4p orbitals on the other.
Atomic Number 47 Element
This will get you
Atomic Number 47 Sterling Silver
Now do the same for the 4d and 5s orbitals
The thing to remember here is that in silver's case, the 4d orbitals will be completely filled. That implies that you won't have two electrons in the 5s orbital, since one will be kept in the lower 4d orbitals.
This means that the electron configuration of silver will be
Using the noble gas shorthand notation will get you
Related questions
