The elements calcium and strontium have similar chemical properties because they both have the same. A.)Number of completely filled sublevels B.)Mass number C.)Atomic number D.)Number of valence electrons. Element Strontium (Sr), Group 2, Atomic Number 38, s-block, Mass 87.62. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images.

- Electronegativity Chart
- Lead Electron Configuration
Electron Configuration For Strontium
What is the Electron Configuration of Strontium?
Strontium Atomic Number
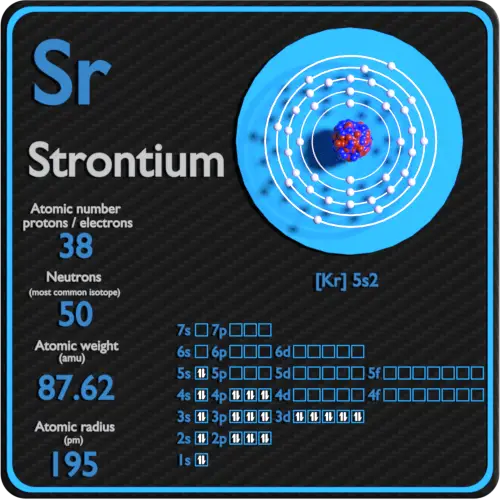
Strontium Atomic Number And Mass
How Many Valence Electrons Does Strontium Have
Lower Atomic Number Strontium
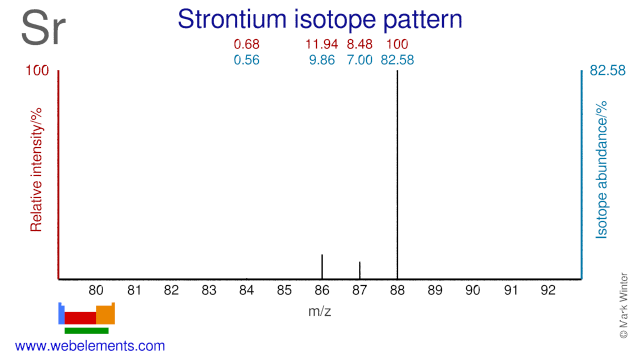
Strontium Atomic Number And Symbol
Strontium Number of Valence Electrons
